The US FDA has authorized donanemab for the remedy of Alzheimer's illness, which helps the physique take away the hallmark plaques that accumulate within the mind, resulting in dementia.
Donanemab, manufactured by Indianapolis-based Eli Lilly, is a monoclonal antibody remedy designed to gradual the development of early signs of Alzheimer's and scientific trials have proven that the remedy slowed cognitive decline in sufferers by as much as 35 p.c.
Now that it's authorized, Eli Lilly stated it is going to be bought below the title Kisunla and administered by IV infusion as soon as a month. It would value $695 per vial — that's about $32,000 yearly.
Though it is a main breakthrough within the remedy of Alzheimer's, consultants have raised issues about donanemab, as it could pose a threat of bleeding within the mind, which led to the demise of a number of sufferers throughout the trials.

Donanemab, manufactured by Indianapolis-based Eli Lilly, is a monoclonal antibody remedy designed to gradual the development of early indicators of Alzheimer's illness
Donanemab can be bought below the title Kisunla and can value $695 per vial — that works out to about $32,000 yearly.
Dr. Howard Fillitt, co-founder and chief science officer of the Alzheimer's Drug Discovery Basis (ADDF), Said A press launch stated: 'This approval is one other step towards growing an ordinary of take care of individuals with Alzheimer's illness that may in the end embody an arsenal of progressive therapies, offering much-needed hope to the Alzheimer's neighborhood.
'Diagnosing and treating Alzheimer's earlier than is feasible right this moment has the potential to gradual the development of the illness, giving sufferers invaluable time to take care of their independence for longer.'
Eli Lilly stated the drug slowed cognitive and useful decline in sufferers by 35 p.c in contrast with placebo over 18 months.
This additionally decreased the chance of sufferers reaching superior levels of Alzheimer's by 39 p.c.
Though donanemab has been hailed as a possible remedy, one-quarter of the sufferers in Eli Lilly's trial developed mind swelling and three died from mind swelling or bleeding attributed to the drug.
Moreover, sufferers who acquired donanemab had a barely larger mortality charge – two p.c, whereas sufferers who acquired a placebo had a mortality charge of 1.7 p.c.
As America's getting old inhabitants continues to develop, so do dementia charges. Presently, it's estimated that 6.7 million Individuals undergo from Alzheimer's illness—the most typical explanation for dementia—most of whom are over the age of 65.
It’s estimated that by 2050, this quantity will improve to almost 13 million.
Though the principle explanation for Alzheimer's illness continues to be debated, scientists consider the harm might be the results of an irregular build-up of proteins — amyloid and tau — in and round mind cells.
In Alzheimer's sufferers, amyloid proteins should not cleared from the physique successfully and finally kind plaques within the mind. Tau proteins detach from neurons and kind tangles.
Each of those may cause neuron demise, making it troublesome for alerts to be transmitted all through the mind.
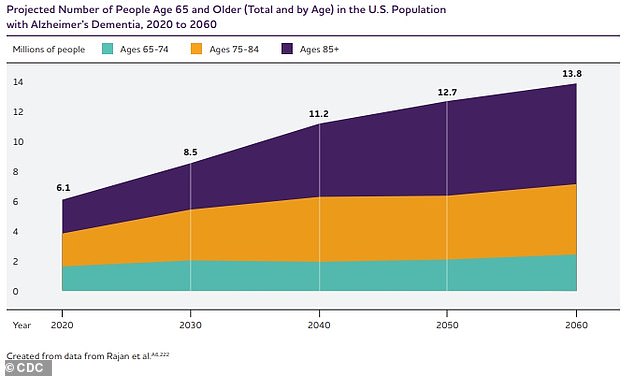
The above graph exhibits the projected estimate of Alzheimer's illness sufferers within the US by 2060
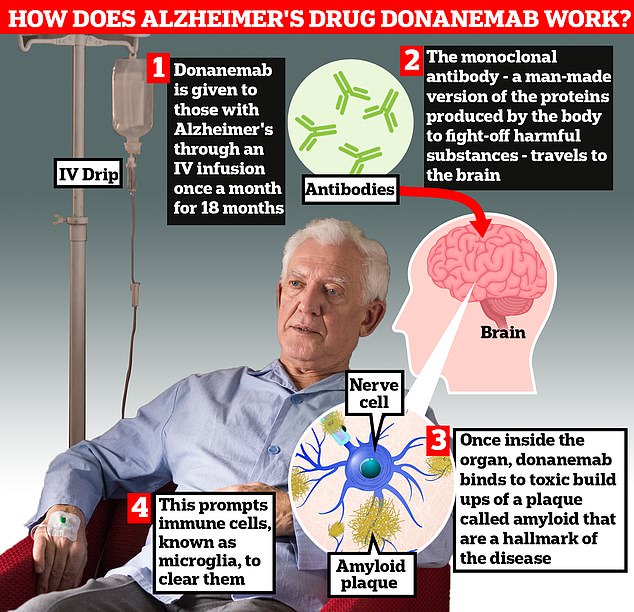
Donanemab is designed to focus on amyloid. Upon coming into the physique by way of IV, the monoclonal antibody travels to the mind.
As soon as contained in the organ, donanemab binds to the poisonous buildup of amyloid plaques, which This prompts immune cells referred to as microglia to clear them up.
Sufferers cease taking donanemab as soon as the amyloid deposits are cleared from the mind.
This remedy is for individuals with early symptomatic Alzheimer's illness or gentle cognitive impairment.


